In our last post, we saw
how crucible steel was manufactured after around 1740 or so, using the process invented by Benjamin Huntsman. While crucible steel was a significant improvement over
blister steel in terms of quality, it was still somewhat expensive to produce. Therefore, many firearm manufacturers used steel for smaller parts, such as sear springs, frizzens etc. and many barrels were still made of wrought iron, instead of steel. As we saw in our previous post, some larger manufacturers like Remington and Colt did offer superior steel barrels after 1820 or so, but they cost over double the price of wrought iron barrels and therefore, both companies sold wrought iron barrels as well, as a cheaper alternative to their steel barrels. High end firearm manufacturers combined steel and iron to make
damascus barrels. These were beautiful to look at, but they were expensive to produce and generally designed for rich clients.
So what was the reason for the higher cost of steel. Well, let's look at the processes involved to convert iron ore to steel using the crucible steel method, as done before the 1850s:
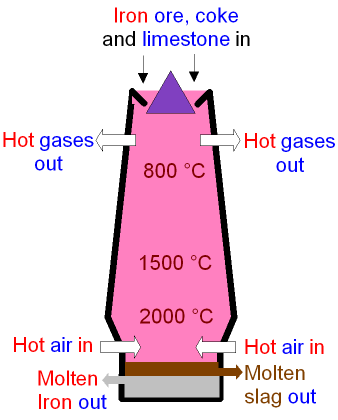
- Convert the iron ore to pig iron or cast iron, using a blast furnace.
- Convert the cast iron into wrought iron, using a finery forge, or later on, a puddling furnace.
- Convert the wrought iron into blister steel, using the cementation process.
- Convert the blister steel into crucible steel. using the Huntsman process.
All four steps needed to be done to produce crucible steel, whereas producing wrought iron only required the first two steps. Steps 2, 3 and 4 also required skilled workers with specialized training (we studied about specialized workers called
puddlers,
puller-outs and teemers in the last few posts). Step 2 was also not geared towards mass production. Using finery forges was a slow process and work-intensive in nature. While the puddling forge replaced the finery forge, it also required specialist workers and puddler workers generally had short life spans as well, due to the unhealthy and stressful nature of their work. Step 3 took wasn't a continuous process either and took the longest time to finish (typically, a batch would take 2 weeks to convert from wrought iron to blister steel). Step 4 was also done in batches, since it was limited by how much puller-outs and teemers could lift at a given time. Step 4 also typically took around 4 hours to finish. No wonder, crucible steel/cast steel cost so much more than wrought iron.
Improvements in the crucible steel manufacturing process, done in the United States in the middle of the 19th century, rendered step 3 unnecessary, as it was now possible to convert wrought iron to crucible steel directly in the crucible. However, the improved process still took a few hours to accomplish, was still a batch process and required skilled workers. Therefore, wrought iron was still the material of choice for many gun makers. Incidentally, large construction projects like bridges and towers of this era also generally used wrought iron, because of the non-availability of large volumes of steel to meet the demand.
The price of steel did not drop until an English engineer named Henry Bessemer invented the
Bessemer process in 1856. With his invention, the cast iron produced in step 1 above could be directly converted to quality steel, without going through steps 2, 3 and 4. It could also be produced in larger volumes than using the crucible process and could be done in 30 minutes, further reducing costs. In fact, the steel produced by his method cost the same price or cheaper than wrought iron. Since steel is generally harder and tougher than wrought iron, after this low-cost production method was invented, most industries stopped using wrought iron altogether and switched to steel completely. In fact, in today's modern world, the only people producing wrought iron are traditional blacksmiths in tiny shops employing only one or two people. We will study how the Bessemer process worked in today's post.
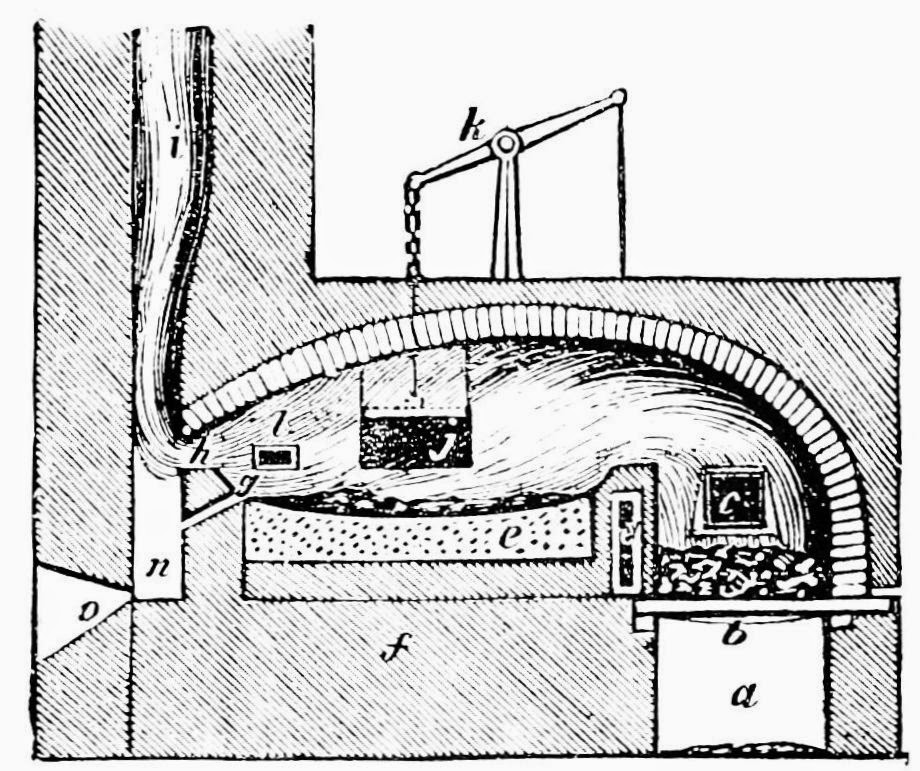
The process consists of melting cast iron in a large vessel (called a
Bessemer converter) and blowing air through the molten iron from the bottom of the vessel, through nozzles called "tuyers". The oxygen in the air oxidizes impurities such as silicon, manganese and excess carbon and forms oxides, which either escape as gases or form lighter slag which floats on top of the molten iron and can be separated. The oxidation of impurities also raises the temperature and keeps the iron in a molten state. The materials used to line the insides of the Bessemer converter vessel also play an important part in removing some impurities, as we will see below. The production of oxides causes a large flame to appear in the mouth of the vessel and monitoring this flame gives an indication of how the oxidation process is proceeding. After the oxidation is complete, the slag is removed and a precise quantity of carbon and other elements are mixed into the molten metal to form steel. This molten steel is then poured into molds to solidify.
![]()
A Bessemer converter. Click on the image to enlarge. Public domain image.
The process of converting cast iron to steel only takes about 20 to 30 minutes and doesn't use as much coke as some of the other processes we've studied in the past. Also, large vessels can be built to handle about 30 tons of metal at a time, making it more efficient for producing large volumes of steel. Typically, a factory has at least two converter vessels for efficiency, so that while one vessel is being filled or emptied, the other one is busy melting the iron ore.
The process of oxidizing iron (decarburizing) with forced air was actually known to people outside Europe, many centuries before the Bessemer process was invented. We know that the Chinese had a decarburizing process in the 11th century AD and there are European traveler accounts of Japanese using a similar process in the 17th century. However, they produced steel in smaller quantities only. It was Henry Bessemer, who converted this process into a large scale industrial production process and we therefore know it as the Bessemer process.
The invention of the Bessemer process was due to a lucky accident. The Crimean war had started and Henry Bessemer happened to meet King Napolean III in 1854 in Vicennes, France and had a short conversation with him, where the King said that what the world needed was for someone to invent a better and cheaper way to produce steel in quantity, so it could be used for guns (both firearms and cannon were largely made of wrought iron at this time). Henry Bessemer started working on the problem in 1855 and patented the process in 1856. A lucky discovery by him actually gave him an insight into the process. He was working with a puddling furnace and by chance, some of the wrought iron pieces ended up on the side of the puddling chamber and were exposed to the furnace's heat for a while. When he went to push those pieces back to the middle, he discovered that the pieces had been converted to steel. This gave him the idea to rework the furnace to push high pressure air via pumps through the iron. "But wait a minute", the reader asks. "Won't blowing air on top of an object cool it down? People blow air via their mouths to cool down hot coffee or hot soup, so why doesn't blowing air cool down the iron?" Well, hot coffee or hot soup don't contain impurities that burn, whereas cast iron does. The oxygen in the air causes the impurities to burn, which increases the temperature of the vessel, which in turn burns more impurities and increases the temperature of the vessel even more, until the iron melts completely. The first impurities to burn are the silicon and carbon in the pig iron, followed by the rest of the impurities.
In order to make the process more popular, Bessemer licensed his process out to four different vendors in different geographic areas, with the plan of gaining market share for his method. He sold the process to the four vendors for a total of £27,000, but none of them could make it work successfully and he ended up getting sued in court! In the end, he bought back his patent licenses for £32,000 and built his own factory. In his initial process, his method consisted of burning off just enough impurities to reduce the carbon content to the required amount to make the grade of steel desired and then stopping the flow of air. Well, that was the theory anyway, but it didn't work so well in practice and he spent large sums of money unsuccessfully trying to figure out how to determine when to stop blowing the air. Another issue was that certain impurities in the steel also react with nitrogen gas, which happens to be a large part of air as well.
It was left to another British metallurgist called Robert Mushet to provide the solution. Before the Bessemer process was invented, Robert Mushet had discovered in 1848, that adding a small amount of spiegeleisen (an alloy that is rich in carbonates of iron and manganese mainly, with a little carbon and silicon as well) to steel made it much easier to work with when heated. The sample of spiegeleisen was brought back to him by a friend who had returned from a tour of the Rhineland area in Germany and thought that he might like to look at the shiny mineral (spiegeleisen is very shiny and the name literally means "mirror iron" in German). We now know that
adding manganese to steel has the effect of increasing the malleability of steel, as we saw earlier when we first started this series.
A sample of Spiegeleisen. Click on the image to enlarge. Public domain image.
Shortly after the Bessemer process was invented, another friend, Thomas Brown, knowing of Robert Mushet's interest in metallurgical problems, brought him a sample of poor quality Bessemer steel and challenged him to improve it. His solution was very simple and was overlooked by everyone else, including Henry Bessemer. Instead of trying to determine when the level of carbon content in the steel had reached the required level and then stopping the flow of air, he instead kept pumping in more air until the entire content of carbon and other impurities had burned off. After all the impurities had been burned off, that's when he stopped the flow of air and added a precise amount of spiegeleisen back into the molten iron, to add back the required amount of carbon and manganese and form high quality steel. This improvement made it much easier to produce steel rails and bars. He also invented other processes to improve the casting of steel (his method is still used today) and also developed the first true modern tool steel. Robert Mushet dreamed that he and Bessemer would become rich men by his inventions, but he didn't manage to profit by them at all, whereas other people did. By 1866, he was bankrupt and ill and his 16 year old daughter went to London alone and angrily confronted Henry Bessemer in his private office and told him that he wouldn't have become rich without her father's invention. Henry Bessemer saw the logic in her argument and paid Mushet a pension of £300 annually (which was a big sum of money in those days) until he died in 1891.
There was also another problem with Henry Bessemer's process. Well, it really wasn't a problem for him, because he was in England and English iron was low in phosphorus content. Remember the section above, where we mentioned that the lining of the Bessemer converter vessel also plays a role in removing some impurities from cast iron. Bessemer lined his vessel with clay and it worked very well with cast iron with low phosphorus content. The process using a clay lining is called
acid Bessemer. The trouble is that in the rest of Europe, their cast iron contained a larger amount of phosphorus and this impurity wasn't removed by the clay lining, which resulted in low-grade steel being produced (phosphorus weakens steel). A British chemist by the name of Sidney Gilchrist Thomas solved this problem in 1876, with the help of his cousin, Percy Gilchrist. His solution to the problem was to coat the inside of the vessel with a lining of dolomite or limestone, which removes the phosphorus impurities. This process is called the
basic Bessemer process, as the lining is alkaline in nature (as opposed to the acid nature of the clay lining). It is also called the
Gilchrist-Thomas process, after its inventor. The process actually generates more slag than the acid Bessemer process. As an extra bonus, the high phosphorus content of the slag meant that it could be sold to farmers as a fertilizer, thereby increasing the profit of the factory! The invention of the basic Bessemer process was very valuable to European countries like Germany and Belgium, where the iron had high phosphorus content and Thomas' name became much more well-known in those countries than in his native England! In the United States, even though more iron ore is low in phosphorus, his method still found lots of supporters here too.
The Bessemer process quickly made Sheffield a major producer of steel. In America, a team of investors went over to England in 1863, to license the technology, with a view to using it to improve shipbuilding, armor and armaments. They built their first factory in Troy, New York, in 1865, to manufacture steel rails for trains. The main American engineer involved, Alexander Holley, continued to improve the Bessemer process and built or consulted for about a dozen different steel plants between 1866 and 1877, including the first Pennsylvania Steel plant for the Pennsylvania railroad company. An early investor who saw great potential in the improvements made by Holley was Andrew Carnegie. who hired Holley to build the Edgar Thomson Steel Works in 1873, located in Pittsburgh. This was one of the largest steel plants in the country at that time and helped make the United States a world leader in steel production, overtaking Britain by 1890 or so. Manufacturing steel made Andrew Carnegie one of the richest men in America and towards the end of his life, he donated his vast fortune to various causes, including funding thousands of public libraries and some universities (he's well known for his contributions to Carnegie Mellon University, but what is not as well known is that he also donated large sums of money to the Tuskegee Institute in Alabama and the University of Birmingham in England). The Edgar Thomson plant is still in service, now part of US Steel, and this factory currently produces about 28% of US Steel's production in America. About 900 people work in here, many of whom had fathers, grandfathers and great-grandfathers working in the same factory as well.
With the invention of the Bessemer process, not only did the time taken to produce steel from pig iron drop significantly (it was faster to produce than even wrought iron!), it was more efficient and could work with larger volumes of cast iron as well. The cost of producing good-quality steel dropped from about £60 per ton to about £7 per ton, shortly after Bessemer started his first factory. With improvements to the process made by others, the prices dropped even more. For instance, an invention by William Jones, while working in the Edgar Thomson steel plant, improved the Bessemer process to become a continuous process. flowing molten iron directly from the blast furnace to the bessemer converter, without waiting for the cast iron to harden first. As a result of this, steel began to replace wrought iron in many applications, as it was now cheaper to produce, as well as being tougher and stronger than wrought iron. The Bessemer process started declining in England around 1895, but it continued in other places in the world for a lot longer. Germany produced most of its steel in the 1950s and 1960s using this process, and in America, the last factory using the Bessemer process closed in 1968. One of its issues was actually its speed of production -- it ran too fast! Given that the steel could be produced in under 20 minutes, this gave little time to analyze the steel and make sure that it has the alloying elements in the correct proportions and to adjust the percentages as needed. One of the later improved Bessemer processes (the oxygen lance process) replaced the Bessemer process in many places. The oxygen lance process blows pure oxygen instead of air, over the molten metal, to better improve oxidation. Interestingly, the oxygen lance method was actually patented by Henry Bessemer in the 19th century, but he could never build it with the available 19th century technology, because of the difficulty of obtaining large quantities of oxygen.
We will study some more improvements in steel making in the next few posts.